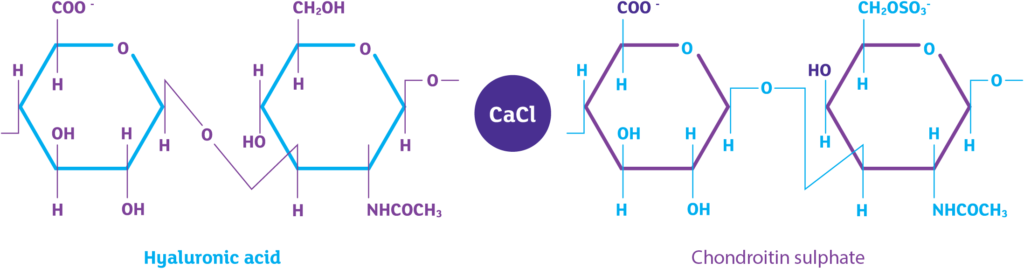
- HA Hyaluronic acid 800mg/50ml (1.6%)
- CS Chondroitin sulphate 1g /50ml (2%)
- CaCl Calcium chloride (0.87%)
What is iAluRil used for?
Effective treatment of:
- Bladder Pain Syndrome / Interstitial Cystitis
- Radiation Cystitis (irradiation of pelvic tumours)
- Chemical Cystitis (BCG)
And relief and prevention of:
- Recurrent Bacterial Cystitis (rUTIs)
What comes in the box?
- Prefilled syringe containing 50ml of iAluRil
- Plunger to be screwed onto the end of the syringe
- Luer-lock adapter for attaching to an intermittent catheter
- iAluadapter® to attach to the end of the syringe for catheter-free instillation of iAluRil
iAluRil active ingredients
Sodium Hyaluronate (HA)
Chondroitin Sulphate (CS)
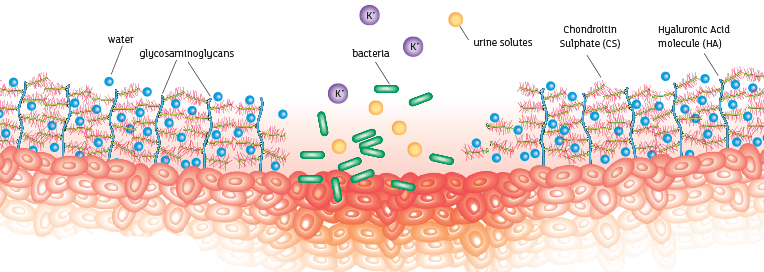
These elements bind together with water molecules to create a waterproof protective lining to the inside of the bladder¹.
Calcium Chloride (CaCl)
The addition of calcium chloride is what makes iAluRil different.
Calcium chloride stabilises the iAluRil solution and provides superior adhesive and protective properties¹⁻³.
Restoring a healthy bladder
What makes iAluRil different?
Combination GAG therapy
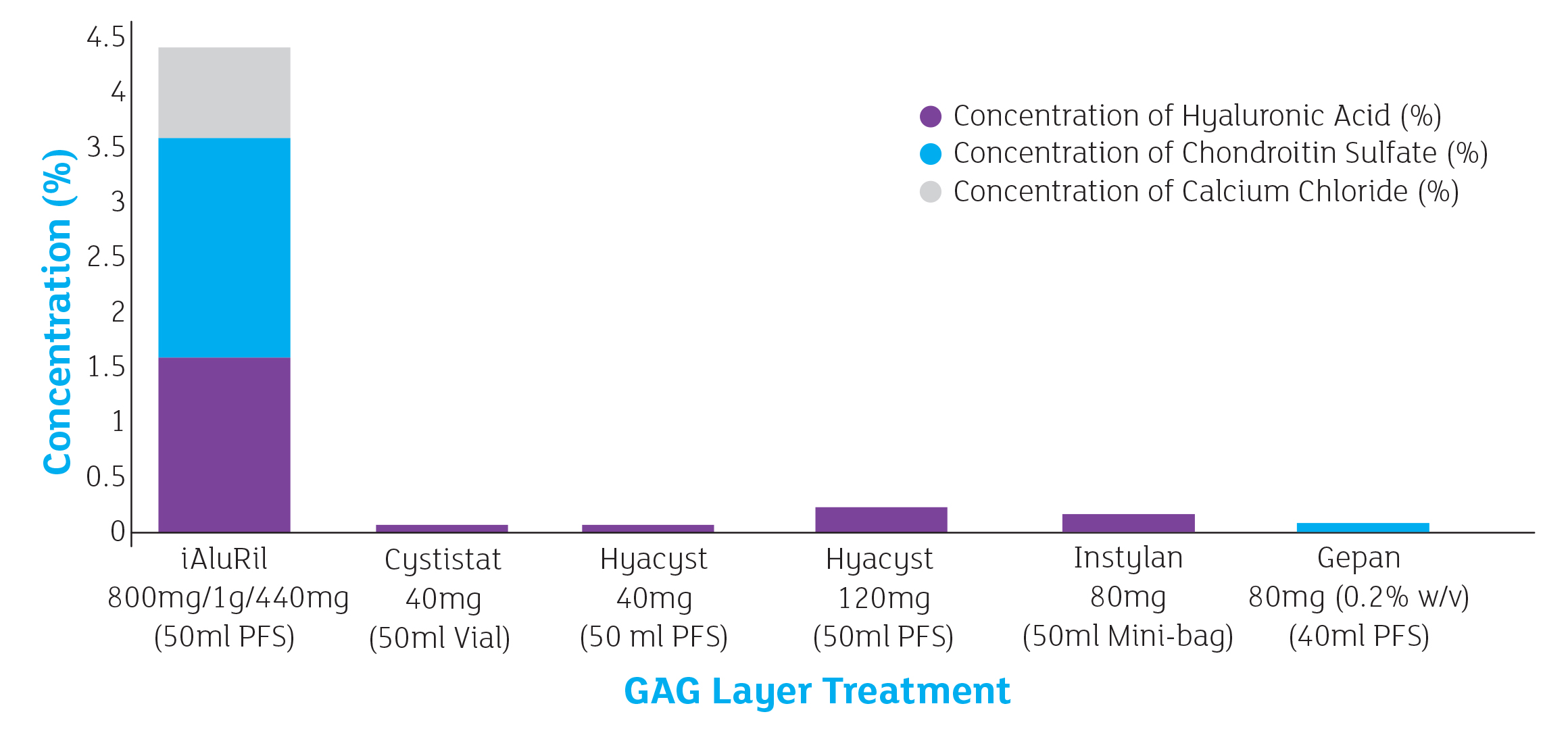
As a healthy GAG layer is made of more than one component, it follows that an effective therapy will contain a combination. iAluRil is the only GAG therapy to contain both HA and CS; a combination which allows a more effective replenishment of the GAG layer.
The only 3 component GAG therapy
Not only is iAluRil the only combination GAG therapy, it is also the only one to contain 3 components. The addition of
calcium chloride (CaCl) provides:
Stability:
Calcium (Ca2+) ions help stabilise sodium hyaluronate (HA) and sodium chondroitin sulphate (CS) in the solution³⁻⁴
Persistence:
iAluRil is able to adhere to the bladder epithelium due to its mucoadhesive properties²
Functionality:
Calcium ions support movement of electrolytes, helping to maintain the protective effect of the GAG layer ¹’³
Viscosity:
iAluRil’s optimised viscosity profile supports ease of instillation⁵
Evidence-based and clinically proven
iAluRil has the most evidence of any GAG therapy, with the most clinical studies and
high quality data from a range of different types of trials.
- 1st in class with level 1b data and over 70 publications⁶
- The only GAG therapy with comparative data vs. multiple antibiotic prophylaxis regimens⁷⁻⁹
- The only GAG therapy with multiple published, placebo controlled trials⁶’⁹
References
Reference 1
Hurst, Robert E et al. “Functional and structural characteristics of the glycosaminoglycans of the bladder luminal surface’’ The Journal of Urology vol. 138 (1987) 0022-5347/87/1382-0433
Reference 2
Stellavato, Antonietta et al. “Hyaluronic acid and chondroitin sulfate, alone or in combination, efficiently counteract induced bladder cell damage and inflammation.’’ ESSM Poster, Ljubljana 2019
Reference 3
Gribbon P, Heng BC, Hardingham TE. “The analysis of intermolecular interactions in concentrated hyaluronan solutions suggest no evidence for chain-chain association.’’ Biochem J. 2000 Aug 15;350 Pt 1:329-35.
Reference 4
Horkay, Ferenc et al. “Chondroitin Sulfate in Solution: Effects of Mono- and Divalent Salts.” Macromolecules vol. 45,6 (2012): 2882-2890. doi:10.1021/ma202693s
Reference 5
Zoppetti G, Puppini N, Pizzutti M. 2006 May 26; “Compositions comprising glycosaminoglycans of low viscosity and use of said composition in therapy of chronic cystitis’’ EP2034956B1
Reference 6
Damiano R, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic Acid and chondroitin sulphate: a placebocontrolled randomised trial. Eur Urol. 2011
Apr; 59(4):645-51. Epub 2011 Jan 18
Reference 7
Gugliotta et al. Is intravesical instillation of hyaluronic acid and chondroitin sulphate useful in preventing recurrent bacterial cystitis? A multicentre case
control analysis. Taiwanese Journal of Obstetrics and Gynaecology 2015; 54:537-540
Reference 8
Torella M et al. intravesical therapy in recurrent cystitis: a multicentre experience. Journal of Infection and
Chemotherapy 2013; 10.1007/s10156-013-0609-6
Reference 9
De Vita D, Long-term efficacy of intravesical instillation of hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: 36 months’
follow-up. Clin.and Exp. Obstet. & Gynecol. – CEOG XLV n.2,2018
IAL1010244C2_DEC2023